HitGen's chemistry business adheres to the philosophy of high quality, efficiency and continuous innovation, providing global partners with effective and competitively cost-efficient chemical solutions.
Computational Sciences and Informatics
Computational chemistry uses physics-based algorithms and computers to simulate chemical events and calculate atoms and molecules' chemical properties, which can significantly accelerate the lengthy and costly drug discovery process. HitGen computational science team includes several experts from bioinformatics, cheminformatics, computer-aided drug design (CADD), and AI-aided drug discovery (AIDD), which can offer a series of services to support your drug discovery project during all phases.
HitGen leverages its deep expertise in library design and an ever-growing repository of compound structures to offer a robust, unique, and cost-effective compound library for your drug development projects. Using cutting-edge algorithms and top-tier cloud computing, we quickly and accurately pinpoint promising compounds to accelerate drug discovery.
l Extensive Databases & Multi-dimensional Screening:
By combining a commercial compound database of over 8 billion structures with our proprietary collection exceeding 1 trillion structures, we apply diverse drug-like filters, substructure searches, and similarity searches to rapidly identify potential candidates.
l AI-generated Compound Library Expansion:
Our advanced generative models and graph neural networks create novel, drug-like molecules. We expand the chemical space through scaffold hopping and virtual expansion techniques.
l Receptor-based Virtual Screening:
Capable of screening a wide range of targets—including kinases, GPCRs, phosphatases, and PPIs—our methods integrate existing structural data with AI-optimized receptor models to deliver accurate results even when target structures are limited.
l Ligand-based Virtual Screening:
For targets without complete receptor structures, we utilize pharmacophore models and molecular embedding techniques to efficiently identify candidates, broadening the screening scope and enhancing hit rates.
l High-performance Computing Support:
Leveraging both supercomputing centers and local HPC resources, we accelerate processes from molecular screening to ADMET prediction, ensuring efficiency throughout virtual screening.
Our experienced computational science team supports the entire drug discovery process using structure-based drug design. Advanced AI-driven methods and multi-scale computing enable precise predictions of small molecule binding modes, receptor-guided molecule generation, and free energy perturbation (FEP) calculations. We also excel in complex system modeling and fragment-based drug design.
l Protein Co-crystallization & AI Prediction:
We provide professional protein co-crystallization services and utilize AI to predict co-crystallized models. Combining molecular dynamics with AI-assisted flexible target sampling captures receptor conformational changes, yielding richer dynamic structural insights.
l Advanced Binding Mode Prediction:
We implement various docking strategies, including Induced-fit docking, covalent docking and QM docking, and use 3D-CNN rescoring and AI prediction models to ensure accurate binding mode predictions.
l Receptor-guided Small Molecule Generation:
Based on receptor structures, our AI models and scaffold hopping techniques design novel small molecules that fit precisely into the binding site.
l FEP Calculation Services:
Utilizing enhanced sampling and high-precision simulations, our FEP services accurately assess protein-ligand binding affinities with an average MAE of approximately 1.0 kcal/mol, thus reducing experimental costs and timelines.
l Complex System Modeling:
We model multi-body interactions—such as PROTAC ternary complexes, protein-protein interactions and protein-RNA interactions—to address diverse drug design challenges.
l Fragment-based Drug Design (FBDD):
Through fragment screening and growth strategies, we identify key binding hotspots and transform fragments into lead compounds using structural optimization and AI.

Our advanced ligand-based design techniques deliver efficient and precise support for drug discovery—even when target protein structures are unavailable.
l DEL & AI for Active Molecule Discovery:
Combining DNA-encoded library (DEL) technology with AI screening, we build structure–activity relationship models that predict bioactivity and guide subsequent design and synthesis.
l Intelligent Similarity Search & Clustering:
With a vast compound library and deep data mining expertise, our AI-driven similarity searches and clustering methods expedite the screening of active compounds and lay the groundwork for detailed structural analysis.
l Pharmacophore Model Construction & Optimization:
We create precise pharmacophore models based on known ligands to enhance the accuracy of compound evaluations.
l Novel Compound Design:
Utilizing both in-house and commercial scaffold hopping methods, we refine challenging chemical structures to generate high-value novel compounds.
l Data-driven Activity & ADMET Prediction:
Our AI-driven ADMET and QSAR platform, integrated with experimental data and cheminformatics models, predicts compound bioactivity, toxicity, and ADMET properties. Continuous feedback and reinforcement learning improve prediction accuracy while reducing resource and time requirements.
l Quantum Mechanics & Molecular Dynamics Integration:
By integrating QM-based structure optimization with MD-based conformational analysis, we predict compound stability and flexibility, revealing key binding modes.
In many cases, ligand-based drug design and structure-based drug design can be applied together to develop potent drug candidates. These methods have been involved in different stages and aspects in the research and development at HitGen and benefitted multiple projects.
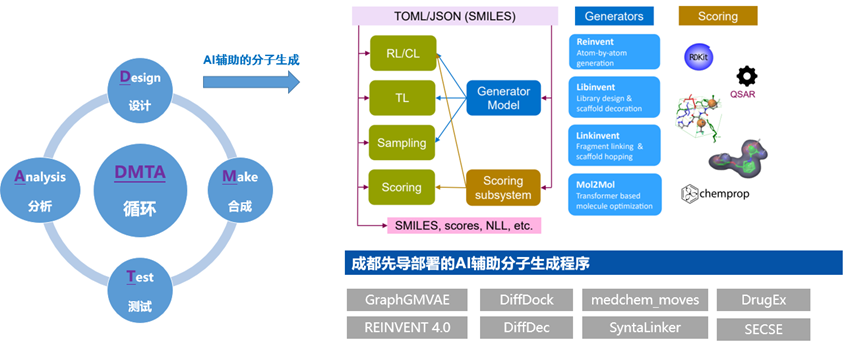
AI-driven Molecular Generation
AI-driven molecular generation is revolutionizing drug discovery and optimization. HitGen employs a diverse suite of AI molecular generation programs that deliver strong capabilities and broad applications.
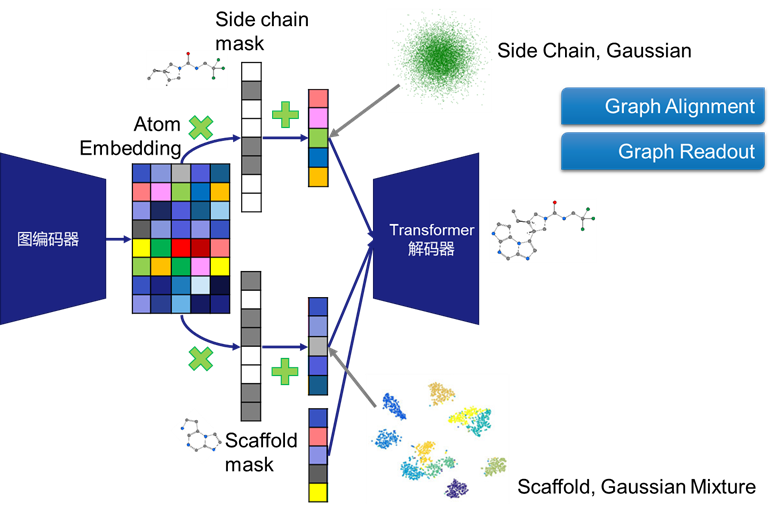
l Scaffold Hopping Algorithm Development with Tencent:
In collaboration with Tencent AI Lab, we developed the GraphGMVAE algorithm, which generates molecules with similar bioactivity but distinct scaffolds while retaining critical side chains. This approach has produced compounds with new patent potential and comparable activity in internal kinase projects.
l Diverse Molecular Generation Programs:
To meet a variety of R&D needs, we deploy over 10 molecular generation programs—including those for random generation, scaffold hopping, R-group replacement, fragment linking, virtual library creation, and receptor-based generation—boosting efficiency and success in drug discovery.
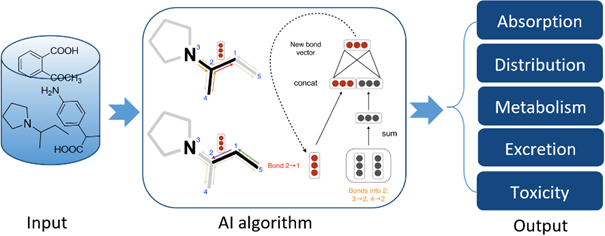
Our AI-driven ADMET prediction platform leverages advanced computational simulations and extensive experimental data to provide integrated assessments throughout compound screening and optimization. This accelerates drug development, reduces risks, and improves candidate success rates.
l Multi-dimensional ADMET Property Prediction:
Our AI models accurately forecast key ADMET characteristics:
1)Absorption & Distribution: BBB permeability, plasma protein binding (PPB), and oral bioavailability.
2)Metabolism & Transport:CYP enzyme-based metabolic stability and transporter-mediated uptake/excretion.
3)Toxicity: Hepatotoxicity, cardiotoxicity (including hERG inhibition), and other safety markers.
This comprehensive evaluation supports early drug assessments and informed candidate selection.
l Adaptive AI Modeling & Project-specific Optimization:
We continuously refine our ADMET models using feedback from internal projects and public databases. For specific targets or project requirements, customized modeling enhances relevance and supports tailored decision-making.
l High-throughput Computing for Large-scale Screening:
By integrating supercomputing centers with local HPC resources, we perform high-throughput ADMET predictions that rapidly assess the drug-likeness of extensive compound libraries, providing swift and precise data support for drug development.
HitGen uses a range of commercial and open-source cheminformatics tools to support better chemical decisions. As the leadership of hit identification platform, we have established a series of capabilities to support our platform to handle scaffold and building block selection, library design, synthesis, selection, and follow-up hit optimization in a time- and space-efficient way.
Well established cheminformatics workflow to do data-driven scaffold and building block selection
Various cheminformatics characterization approaches have been established, such as physicochemical property calculation, fingerprint clustering, and diversity analysis are used to evaluate our proprietary libraries or design new high-quality library
Super-size library (>∼1012 ) structure enumeration, storing, searching, and evaluation
Combining the AI technology and experimental information in the library synthesis and selection to increase the DEL productivity
Data mining for data-driven feature identification and hit proposal
DEL selection data for QSAR model building to predict new compound affinities or activities
We use cookies to provide a better web experience.
By using our site, you acknowledge our use of cookies and please read our Cookie Notice for
More information