HitGen offers comprehensive solutions for the development of oligonucleotide therapeutics, covering the entire process from research and development to production.
Hitston Ltd. (abbreviated as "Hitston") is a global-leading small nucleic acid drug CDMO service platform jointly established by HitGen Inc. (stock code: 688222.SH) and Easton Biopharmaceuticals (stock code: 688513.SH), specializing in providing pharmaceutical companies and research institutions with full-cycle solutions from preclinical to commercial production.
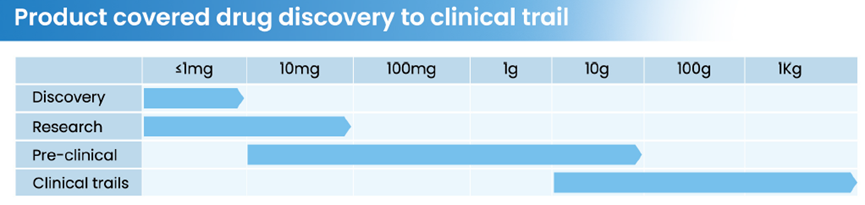
Relying on a 2000㎡ oligonucleotide GMP workshop, 1000㎡ quality control laboratory, and HitGen's professional R&D laboratories, Hitston has achieved gram-to-kilogram-scale API production for IND applications, clinical Phase I-III, and early commercialization stages, with a total investment of nearly 100 million RMB, covering global demand.


The formulation development and production of Hitston is part of an integrated small nucleic acid drug CDMO platform. It has three aseptic formulation production workshops that comply with cGMP standards and has undergone multiple audits by the NMPA, FDA, and the European Union. A single - use liquid - dispensing system is adopted. The filling system is equipped with both vial filling and pre - filled syringe filling production lines. The production environment classification for the vial filling and stoppering machine is B + A grade, and operations such as filling and stoppering are completed within isolators. The batch quantity can be flexibly adjusted according to requirements, with a minimum batch of 500 ml. The liquid - dispensing system of this production line is equipped with a 100L liquid - dispensing tank, a 60L transfer tank, and a 100L aseptic receiving tank. Each tank can be weighed online, temperature - controlled, and protected by nitrogen filling. Aseptic feeding methods can also be used for feeding materials to meet different process requirements. The filling machine is equipped with 6 pump heads, and the production speed can reach 120 bottles per minute. Generally, the filling volume control range can reach ±3%. The pre - filled syringe filling machine is equipped with 10 filling needles and an in - line weighing device for sampling inspection. Generally, the filling volume control range can reach ±5%, and the filling volume range is 1 - 10 ml, meeting the production requirements for nucleic acid formulations in vial or pre - filled syringe packaging.
The professional team conducts rigorous quality control from the initial process assessment to the production stage, and monitors and records in detail throughout the process in accordance with GMP regulations. Its complete cGMP management system provides strong support for the production of high - quality nucleic acid formulations, ensuring project delivery in all aspects and throughout the entire cycle.

We use cookies to provide a better web experience.
By using our site, you acknowledge our use of cookies and please read our Cookie Notice for
More information